
Welcome to your Elite Experience
Track your progress and maximize your Elite benefits.
View your Dashboard
Unlock your Elite Advantages
1
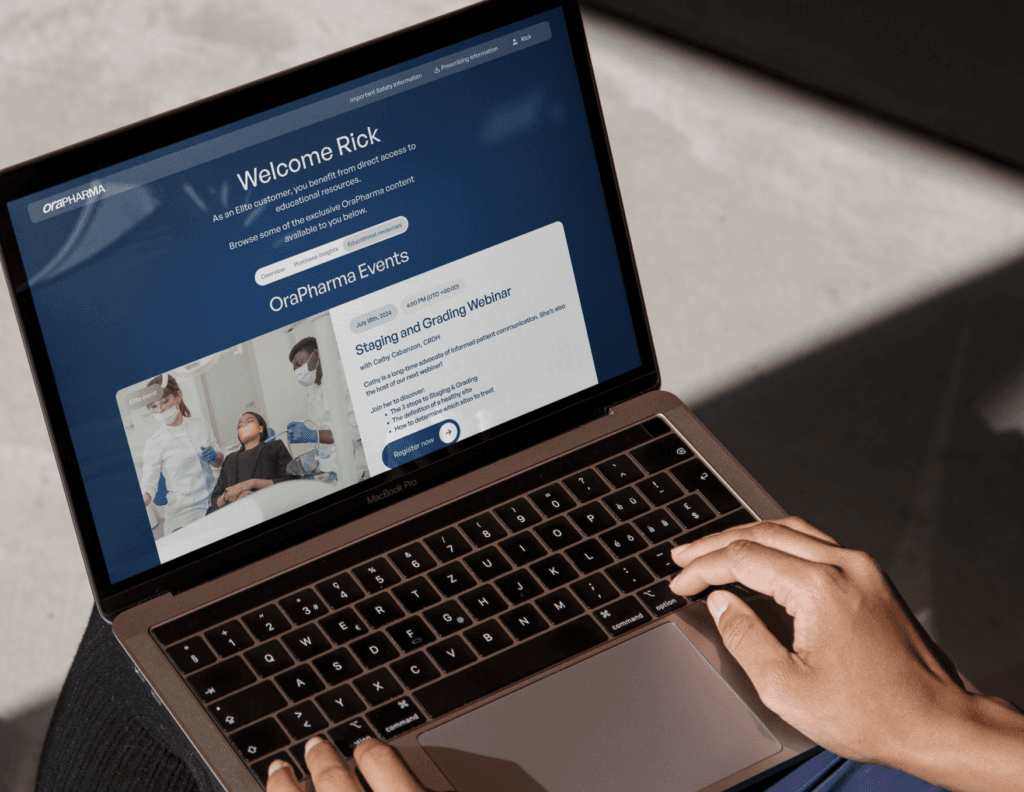
Real-time insights
2
Streamlined purchasing
3
New Discount Tiers
Real-time insights
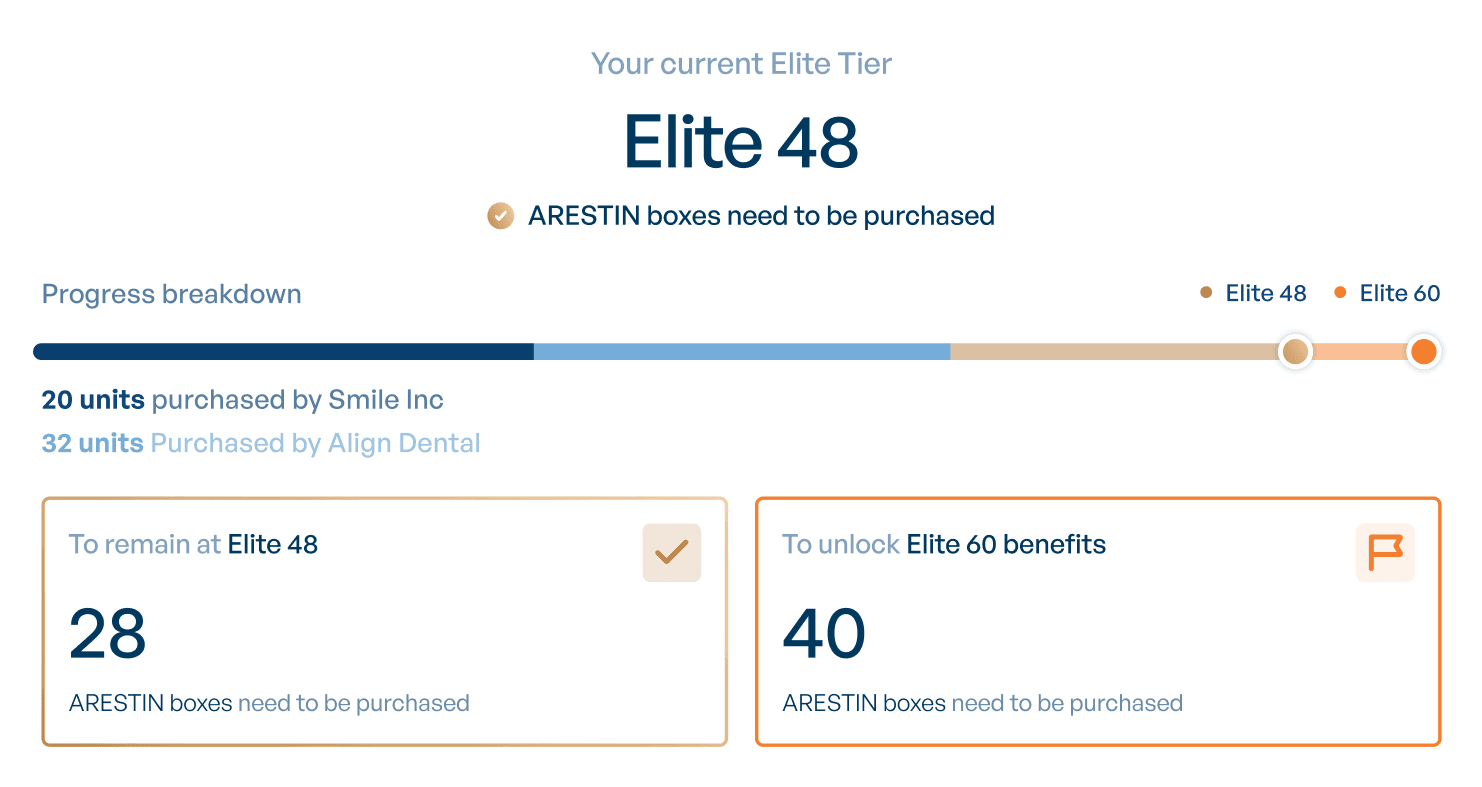
Our dynamic tracking tools allow you to monitor your Elite Tier with data on purchases, progress, and more.
Show me how the Elite Tiers work
Streamlined purchasing
Easily manage your ARESTIN® (minocycline HCl) Microspheres, 1 mg, orders with a clear, organized view of your past and current purchases, ensuring a smooth ordering process.
New Discount Tiers
We've added new Elite pricing tiers and removed our 'one tier a year' limit, so you can now unlock more discounts with fewer restrictions.
Show me how the Elite Tiers work
Elite Program Breakdown
Here is an overview of the tiers
| Elite Tier | Qualifying Volume | 2025 Box Price | Discount |
|---|---|---|---|
| Elite 6 | 6-11 boxes | $656 | 6% |
| Elite 12 | 12-23 boxes | $614 | 12% |
| Elite 24 | 24-35 boxes | $572 | 18% |
| Elite 36 | 36-47 boxes | $537 | 23% |
| Elite 48 | 48-59 boxes | $523 | 25% |
| Elite 60 | 60-95 boxes | $509 | 27% |
| Elite 96 | 96-119 boxes | $495 | 29% |
| Elite 120 | 120-155 boxes | $481 | 31% |
| Elite 156 | 156-299 boxes | $467 | 33% |
| Elite 300 | 300-499 boxes | $454 | 35% |
| Elite 500 | 500-799 boxes | $426 | 39% |
| Elite 800 | 800-999 boxes | $405 | 42% |
| Elite 1000 | 1000+ boxes | $391 | 44% |
Unlock your
Elite Advantages